42 fda health claims on food labels
Questions and Answers on Health Claims in Food Labeling | FDA Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a... FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Authors J Craig Rowlands 1 , James E Hoadley Affiliation 1 Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD 20740, USA. JCRowlands@Dow.com PMID: 16480811 DOI: 10.1016/j.tox.2005.10.023 Food Labeling* Legislation, Food* Nutritive Value Research Design
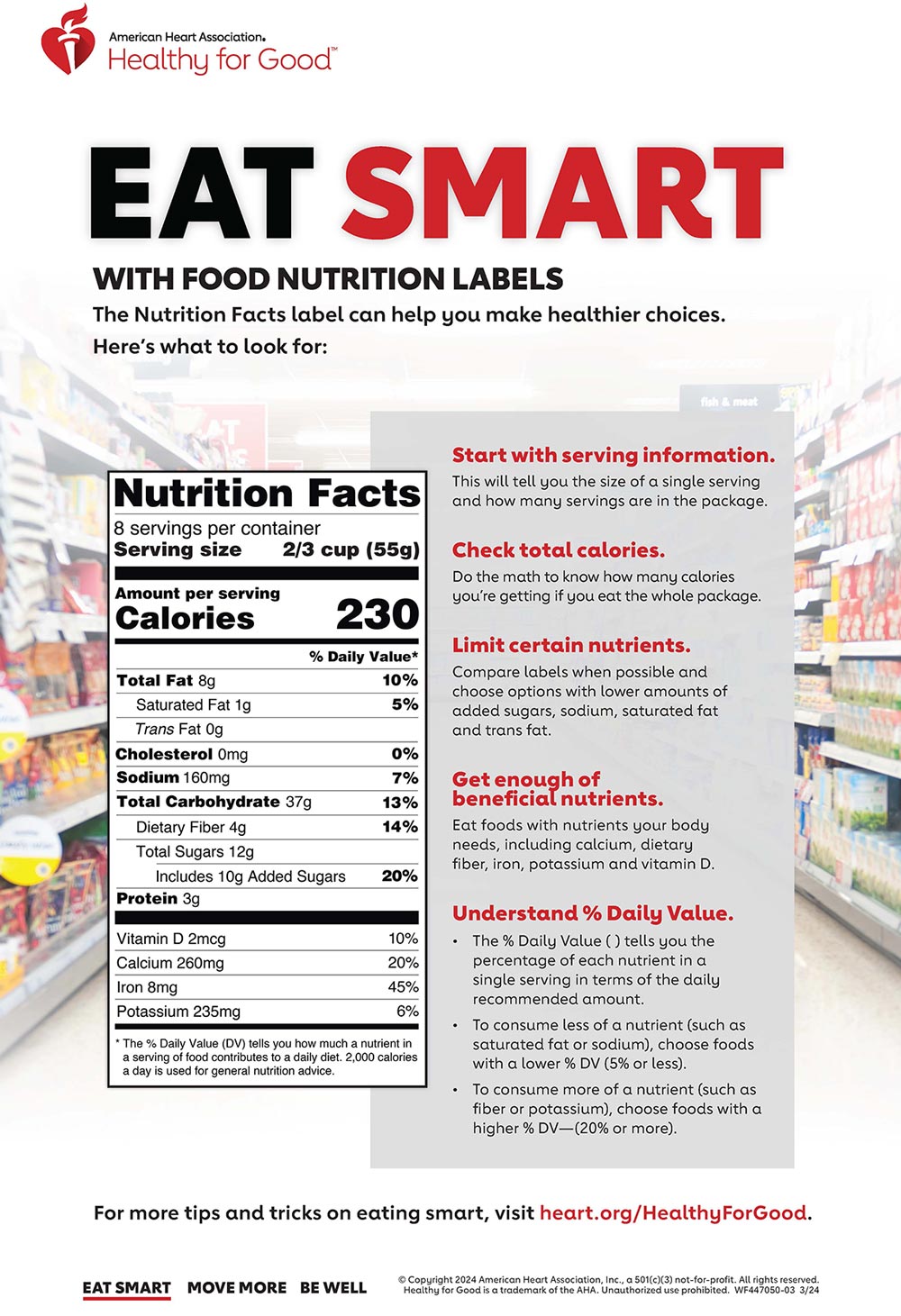
5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease."
Fda health claims on food labels
FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have about three ... 21 CFR § 101.14 - Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes ... What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
Fda health claims on food labels. FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits,... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written... Food Label Claims: What You Can and Can't Trust - WebMD In the U.S., the FDA sets some common definitions for certain food claims. Specifically, the FDA regulates three types of claims. The FDA doesn't always pre-approve these claims. But... Drugs vs. foods - Health claims on food labels - Food labels - Canadian ... Health claims on food labels Drugs vs. foods . Just like the term "food" (definition), the term "drug" (definition) is also defined in the Food and Drugs Act.One difference between foods and drugs is in how they are represented. If a product meets the definition of a food and is regulated as a food, the product is not permitted to carry a drug claim, unless exempted.
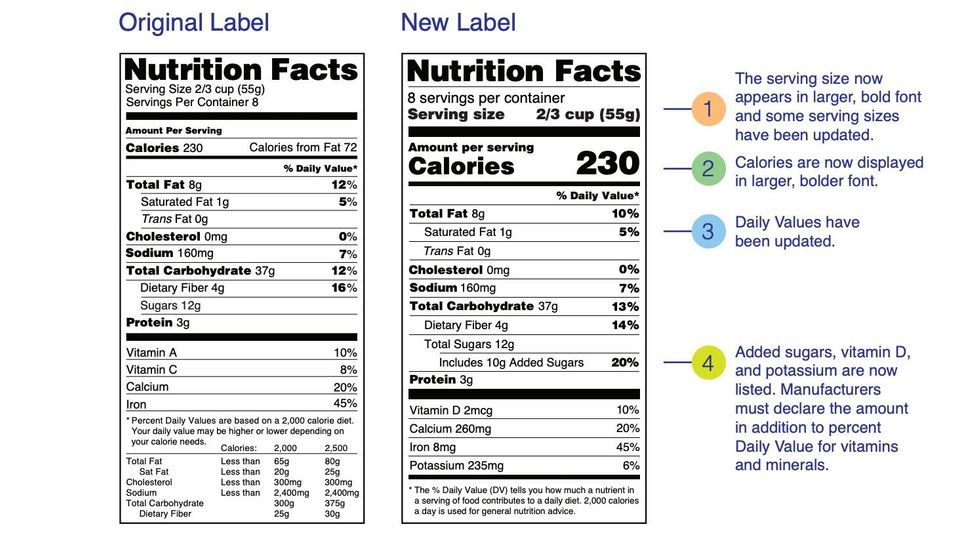
Auburn food historian explains new FDA guidelines for 'healthy' food labels Last week, the U.S. Food and Drug Administration, or FDA, updated its criteria for foods labeled "healthy." The proposed change is based on current nutrition science and prioritizes healthy dietary patterns, continuing from the FDA's overhaul of the Nutrition Facts panel in 2016. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... Everything you need to know about Health Claims on Food Labels The "qualified" health claims. The authorized health claims by the FDA must have significant scientific agreement among qualified experts to support the scientific evidence for a substance - disease relationship. The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and ... FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will...
Function claims - Health claims on food labels - Food labels - Canadian ... When a function claim appears on the label of a prepackaged food or in advertisements made or placed by or on the direction of the manufacturer of the food, the label of a food that is normally exempt from declaring a Nutrition Facts table (NFt) loses its exemption and is required to declare a NFt. See Reasons for losing the exemption [B.01.401. What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims) 21 CFR § 101.14 - Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements (e.g., a brand name including a term such as "heart"), symbols (e.g., a heart symbol), or vignettes ... FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have about three ...
































Post a Comment for "42 fda health claims on food labels"